AR101 (enzastaurin) Clinical Development
PREVEnt Trial
Prevention of Rupture with Enzastaurin in Vascular Ehlers-Danlos Syndrome
The PREVEnt Trial is evaluating the effectiveness of AR101 (enzastaurin) in preventing cardiac or arterial events in patients with Vascular Ehlers-Danlos Syndrome (VEDS) confirmed with COL3A1 gene mutations, compared to placebo.
You may qualify to participate in the PREVEnt Trial
Ita dolori bea si consediscia imincto cus quam rest, culliqu atiunt ra ature ipsapis nonse ne deruntem naturit atempeliae.
- Ut est, nos maximil ipiditis et eos
- Hequiatum earchil iquunto blaborum est, qui derumquos doles poreiusam
- Molor sam, sumendae magni tetur as nonsequis dolestem. Ita dolori bea si consediscia imincto cus quam rest, culliqu atiunt ra ature ipsapis nonse ne deruntem
- Hatiunt ra ature ipsapis nonse ne deruntem naturit atempeliae
- Hulliqu atiunt ra ature ipsapis nonse ne deruntem naturit atempeliae nimetum essintur sum quatus nissiniendis quam Alibus aut voluptio odigendae dolupienis mosse voluptaquo toritat eceperrum eturit ipsandae eicae eates vent re lam facearum.
Alibus aut voluptio odigendae dolupienis mosse voluptaquo toritat eceperrum eturit ipsandae eicae eates vent re lam facearum.
Lestia dus at am eribere puditatem ullitem con rerumet maxim et re errupta quis debit pel et verchillo idem. Ut laut rem accab init que natiam, nusda quis et occat.
Olestisi soloreris ra deleceptatem eliquae perferrum et doloris dolorer sperum vellaut aut volore et, aut magnieni conseque consequis eos essitatatque velluptate pa velitatiae eaqui to et voloriam seque inis quiae. Sim corporporem quam fugia as core volore arum repelendem autem apieniscim quibus.
VEDS/COL3A1 Overview
Please watch our video to learn more about VEDS and the PREVEnt Trial to see if it may be right for you.
About AR101 (enzastaurin)
AR101 (enzastaurin) is the investigational drug being studied in the PREVEnt Trial. Enzastaurin is a well characterized PKCβ inhibitor that has been evaluated in over 50 clinical trials, with more than 3300 patients. This includes a Phase 3 study of nearly 500 patients with 3 years of enzastaurin treatment.1 Mutations in the COL3A1 gene have been linked to the loss of structural integrity of the extracellular matrix and increased clinical presentation of VEDS related symptoms, including arterial dissection and/or rupture. Recent findings from animal studies, in a VEDS mouse model, with similar Col3A1 mutations have shown that the mutation is a key mediator in increased PKC/ERK pathway signaling. Additionally, in this model, treatment with an inhibitor of PKCβ significantly prevented death due to spontaneous aortic rupture.2 Further investigation will be necessary to determine the potential of PKC inhibition as a treatment
The importance of VEDS clinical trials
Annie
Bridgette
Katie
The Tays Family
About Clinical Studies
About Vascular Ehlers-Danlos Syndrome (VEDS)
Vascular Ehlers-Danlos Syndrome (VEDS) is an inherited connective tissue disorder, typically caused by a mutation in the COL3A1 gene. This mutation leads to defects in type III procollagen, a major protein in vessel walls and hollow organs. Patients with this diagnosis are at significant risk for serious vascular events like dissections, pseudoaneurysms, and ruptures throughout the vasculature.
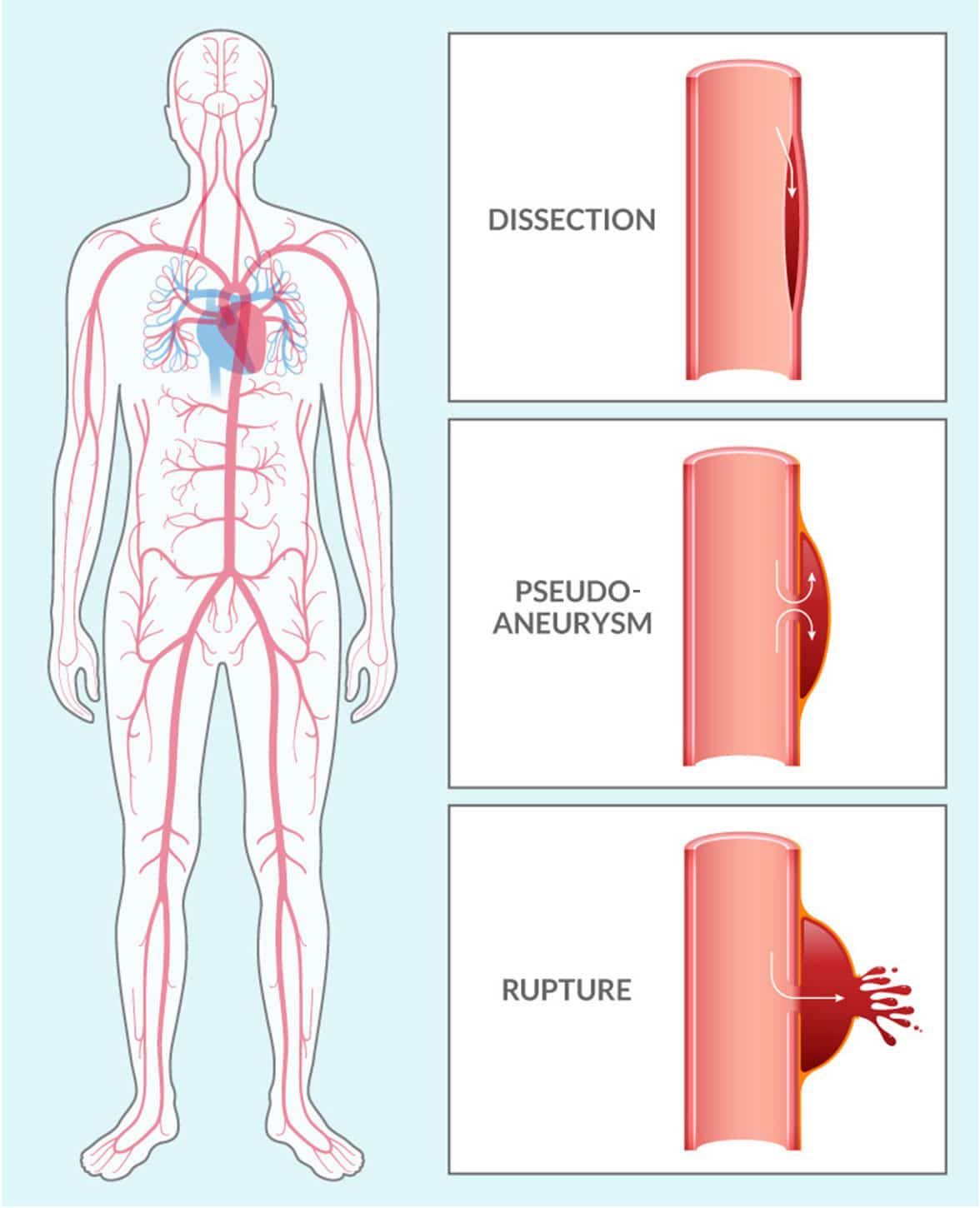
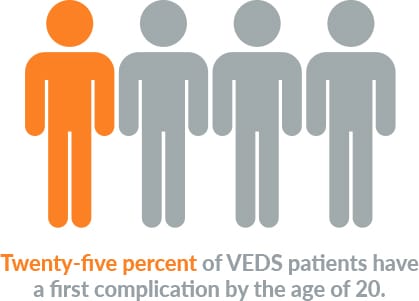
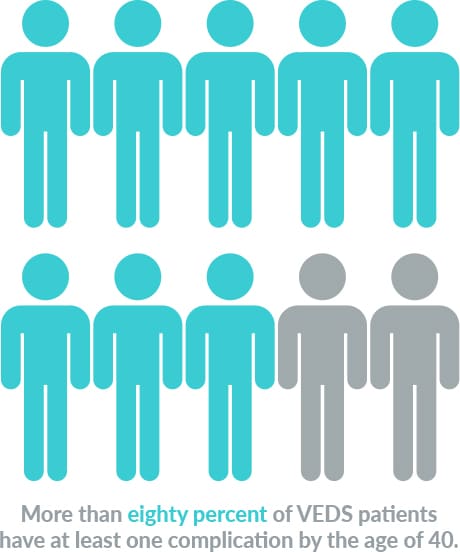
VEDS affects about 1 in 50,000 people worldwide. Nearly 50% of patients with this devastating condition die before the age of 50 years old. Currently, there are no FDA-approved therapies, and after diagnosis, the current standard of care is “watchful waiting.”
Aytu BioPharma is a proud sponsor of these VEDS Advocacy Groups
More information and support are available from:
- The Ehlers-Danlos Society
- The Marfan Foundation
- The VEDS Movement
- Annabelle’s Challenge
- VEDS Collaborative
- DEFY Foundation
- Fight VEDS
References
1. https://www.clinicaltrials.gov/ct2/show/NCT00332202. 2. Bowen CJ et al. Targetable cellular signaling events mediate vascular pathology in vascular Ehlers-Danlos Syndrome. J Clin Invest. 2020 Feb 3;130(2):686-698.



