AR101 – Enzastaurin
About AR101 (enzastaurin)
VEDS/COL3A1 Overview
About Vascular Ehlers-Danlos Syndrome (VEDS)
Vascular Ehlers-Danlos Syndrome (VEDS) is an inherited connective tissue disorder, typically caused by a mutation in the COL3A1 gene. This mutation leads to defects in type III procollagen, a major protein in vessel walls and hollow organs. Patients with this diagnosis are at significant risk for serious vascular events like dissections, pseudoaneurysms, and ruptures throughout the vasculature.
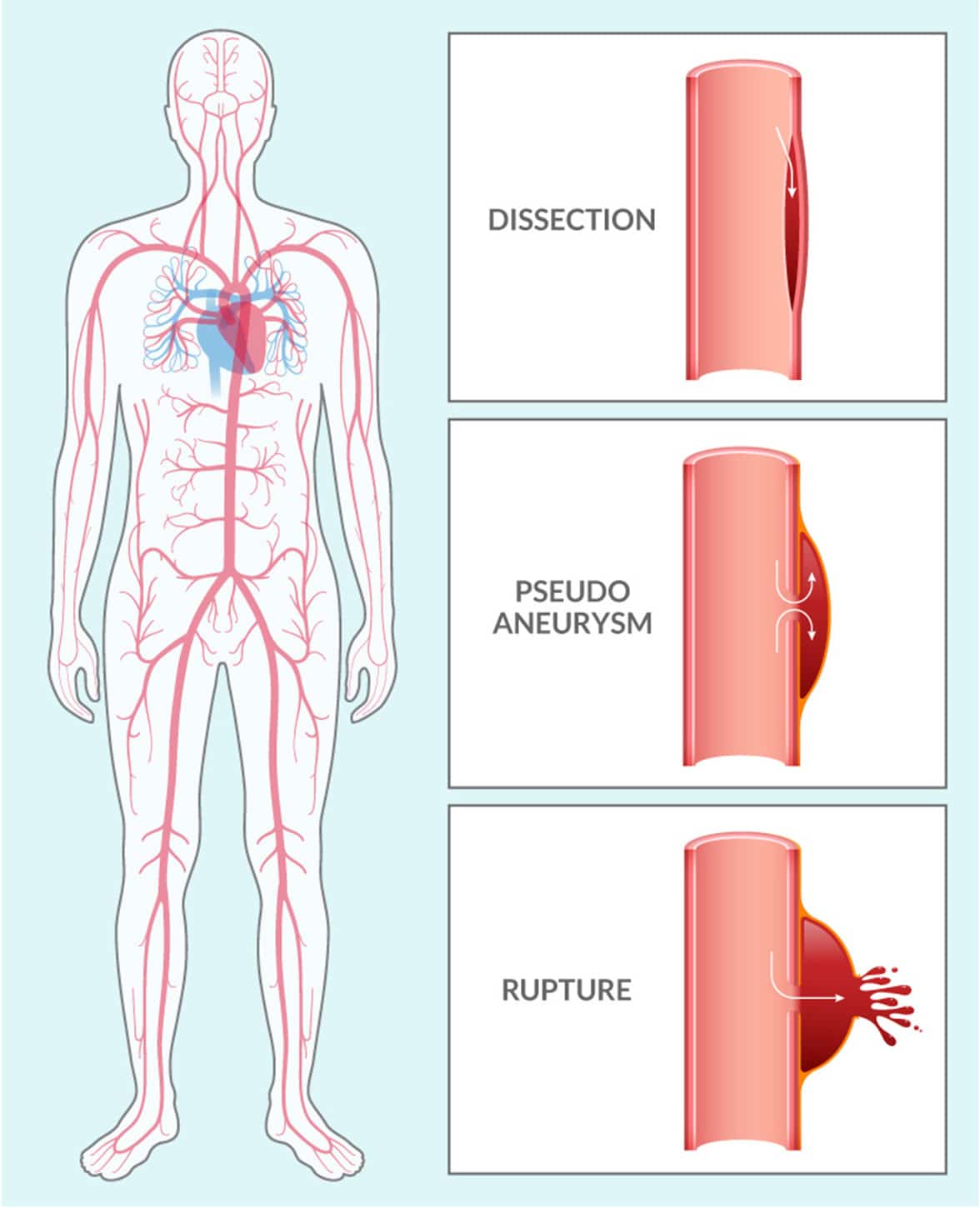
VEDS affects about 1 in 50,000 people worldwide. Nearly 50% of patients with this devastating condition die before the age of 50 years old. Currently, there are no FDA-approved therapies, and after diagnosis, the current standard of care is “watchful waiting.”
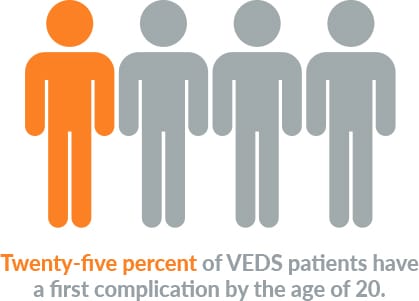
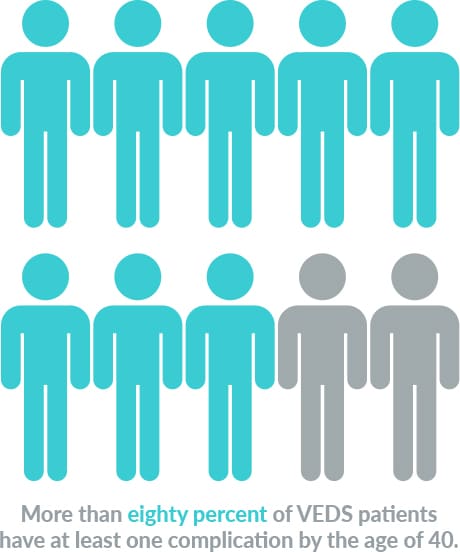
Living with VEDS
Annie
Bridgette
Katie
The Tays Family
References
1. https://www.clinicaltrials.gov/ct2/show/NCT00332202. 2. Bowen CJ et al. Targetable cellular signaling events mediate vascular pathology in vascular Ehlers-Danlos Syndrome. J Clin Invest. 2020 Feb 3;130(2):686-698.
